Laboratory of Macromolecular Crystallography
This is a review of the works carried out in LMC of the IMPB RAS. Information on other papers in this field
may be found in the original papers listed below.
The use of Fourier syntheses histograms for solution of the phase problem
in protein crystallography.
(1986-1993)
The traditional goal of the first stage of determining a macromolecular
structure is to find the function ρ(x,y,z), which presents the
distribution of electron density in the crystal of a studied object. This
function is periodical in the three space directions and may be presented as a
three-dimensional Fourier series
(1)
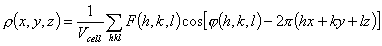
In crystallography, the complex coefficients
F(h,k,l)exp[i φ(h,k,l)] are referred to as
structure factors while real values of F(h,k,l) and
φ(h,k,l) are called magnitudes and phases, respectively. In a
conventional X-ray experiment one can only determine the magnitudes
F(h,k,l). The problem of restoring the phase values is called the
phase problem of X-ray crystallography. Obviously, to solve it some additional
information on the studied object is required. Once approximate phase values
have been found, they (together with experimental magnitudes) may be used to
calculate an approximate density distribution by formula (1).
Additional information on the studied object is often presented as restrictions
on the range of possible electron density values, e.g. ρ≥0, or
ρmin≤ ρ(h,k,l)≤
ρmax. This information is usually incorporated into
iterative "density modification methods" [13]. A more thorough study of the
range of electron density values (or, more precisely, the range of Fourier
syntheses values) is to explore not only the information on which values exist,
but to find how frequently every possible value occurs in the crystal cell.
Below, the distribution of frequencies corresponding to different Fourier
synthesis values is referred to as the synthesis histogram. The analysis of
histograms corresponding to properly phased protein syntheses revealed that
these histograms have a specific asymmetric shape, while the randomly phased
syntheses have a gaussian-type histogram. This histogram property may be used as
additional information in ab-initio phasing [1-3]. An approach was developed to
predict the histogram for as yet unsolved protein structures [15]. The histogram
based information may be used in solving various problems.
The retrieval of unmeasured magnitudes
This approach was used in cases when both magnitudes and phases were unknown for
a part of the structure factors [1-3]. The task formulated was to find such
values for unknown magnitudes and phases which result (together with known
structure factor values for the rest of the structure factors) in a proper
Fourier synthesis histogram.
Phase refinement
In this case it is supposed that approximate values of phases are known. The
goal is a local correction of the phases in order to obtain the best
correspondence of the calculated histogram to the standard one.
Ab-initio solution of the phase problem
A new general procedure for ab-initio solution of the phase problem was
suggested, which was based first on the histogram selection criterion [4]. In
this procedure
- a lot of random phase sets are generated randomly;
- the corresponding Fourier syntheses and their histograms are
calculated;
- the phase sets that had resulted in reasonable histograms are selected for
further analysis.
The analysis of the selected set consists in an attempt to divide the selected
sets into groups of close sets (clusters) and to average phases inside every
separated cluster. An important feature of the cluster analysis and averaging is
a preliminary alignment of the phase sets in accordance with the set of
admissible shifts of the coordinate origin and enantiomorph [11,12].
The relation of the histogram based criteria to other criteria used was analysed
as well [7,10]. In particular, it was shown that many of widely used density
modification methods may be interpreted as an attempt to get phases resulting in
a prescribed histogram [6-10].
The results of the development of this approach were summarised in V.Y.Lunin's
theses [8,9].
March, 24, 2003
V.Lunin
Publications
The full texts of papers
- Lunin, V.Yu. (1986). "The use of information on electron density
distribution in proteins. The retrieval of absent structure factors".
Preprint, NCBI AN SSSR, Pushchino, Russia. (In Russian)
- Lunin, V.Yu. (1988). "Use of Information on Electron Density Distribution
in Macromolecules". Acta Cryst. A44, 144-150.
- Lunin, V.Yu. (1988). "The retrieval of absent structure factors in X-ray
structure determination". Dokl.Akad.Nauk SSSR, 299, 2, 363-366.
(In Russian).
- Lunin, V.Yu., Urzhumtsev, A.G. & Skovoroda, T.A. (1990). "Direct
low-resolution phasing from electron-density histograms in protein
crystallography". Acta Cryst., A46, 540-544.
- Lunin, V.Yu. & Skovoroda,T.P. (1991). "Frequency-Restrained Structure
Factor Refinement. I. Histogram Simulation". Acta Cryst. A47,
45-52.
- Lunin, V.Yu. & Vernoslova, E.A. (1991). "Frequency-Restrained Structure
Factor Refinement. II. Comparison of Methods". Acta Cryst. A47,
238-243.
- Lunin, V.Yu. (1991). "Use of the electron-density-syntheses histograms or
solving of the phase problem in protein crystallography". Preprint,
Pushchino Research Center, Pushchino, Russia.
- Lunin, V.Yu. (1992). "The use of statistical properties of the electron
density Fourier syntheses for the solution of the phase problem in protein
crystallography". Resume of Dr.Sci.Theses, ONTI PNC RAN, Pushchino,
Russia. (In Russian)
- Lunin, V.Yu. (1992). "The use of statistical properties of the electron
density Fourier syntheses for the solution of the phase problem in protein
crystallography". Dr.Sci.Theses, Institute of Crystallography RAS,
Moscow, Russia. (In Russian)
- Lunin, V.Yu. (1993). "Electron-Density Histograms and the Phase Problem".
Acta Cryst. D49, 90-99.
- Lunin, V.Yu. & Woolfson, M.M. (1993). "Mean Phase Error and the Map
Correlation Coefficient". Acta Cryst. D49, 530-533.
- Lunin, V.Yu. & Lunina, N.L. (1996). "The Map Correlation Coefficient for
Optimally Superposed Maps". Acta Cryst. A52, 365-368.
- Podjarny, A.D., Rees, B. & Urzhumtsev, A.G. (1996). "Density modification
in X-ray crystallography". In Methods in Molecular Biology, 56:
"Crystallographic Methods and Protocols", ed. C.Jones, B.Milloy,
M.R.Sanderson, Totowa, New Jersey : Humana Press, 205-226.
|



