Отдел перспективных информационных технологий
Группа компьютерного моделирования наноструктур и биосистем
Multifunctional percolated nanostructured ceramics fabricated from hydroxylapatyte
 European Commission Project NMP3-CT-2003-504937
European Commission Project NMP3-CT-2003-504937
Computational Molecular Nanostructures and Mechanical Properties of Hydroxyapatite
Bystrov V., Paramonova E., Bystrova N., Sapronova A., Filippov S.
Institute of Mathematical Problems of Biology RAS, Pushchino,
142290, Moscow region, Russia
e-mail: bystrov@impb.psn.ru, vsbys@yahoo.co.uk
Hydroxyapatite [Ca5(PO4)3OH; (HAP)] structures have been wide used over past time in medicine as an innate bioactive ceramics materials for bones and dentals implants, because their crystal structure and composition are very analogous with the hard tissues of living bone, corresponds to structure of a mineral bone’s component, that leads to the new bone formation generated by osteoblasts growth on its surface [1-3]. Recently investigations demonstrated that polarized HAP have some specific surface characteristics and provide the additional stimulation of the interaction with living cells [3]. The HAP adhesion properties was improved, if the HAP surface was charged. The mechanisms of polarization and changes of surface electrical and mechanical (adhesion) properties are not yet clear. The arising of the polarization is caused by processes of a charge transfer in the HAP structure. These processes have electrets-like character, because are influenced by electric field and temperature, as was discussed in [4], but haven’t ferroelectric-like type.
In the present work we try to understand the HAP structural changes by using the computer simulation of HAP crystal nanostructures. For these purposes in the given work the computer models are considered and the mechanisms of charge transfer are analyzed on the base of known HAP structural data [5] using the HyperChem and Crystals software [6]. Additionally now we try to use the Gaussian 98 [7] code for development of further HAP structural optimization.
Physical properties of HAP crystal structure, such as ion conductivity and polarization are explained by existence of the channels parallel with c axes. This channel in HAP consists of one-dimensional OH- - chains. The protons occupy the top and bottom party O2- by a casual image with equal probability in hexagonal HAP structure. On the contrary, in the monoclinic phase the direction of a proton in all channels which are taking place in the same plane, parallel plane a, c, is identical: either all upwards, or all downwards. The next plane b/2 is always focused in the opposite party. The ions OH- are surrounded with two pairs next Ca2+ triangles in both structures: both in hexagonal, and in monoclinic [1,2]. The following mechanisms of a charge transfer are probable: polarization and depolarization are attributed to rotation of a proton around OH- in colon-like channels, and the mechanism of proton conductivity based on proton vacancies O2- in ions OH-, where as well as in model of rotation of a proton, proton turns around of an ion O2- in opposite an initial direction. Then the proton moves to the next proton vacancy O2-. When such process proceeds further on OH- to the channels, there is a proton transfer on the large distances in the channels. The large relaxation times are attributed to the large distance of the OH- - OH-. The polarizing charge under the polarization process by means of the proton transport depends on the number of migrated protons and extent of migration [3]. Both these parameters are controlled by the temperature and electric field.
For the first stage of the HAP structure transformation, charges transfer and HAP surface polarization we explore the initial HAP structure in this work. We’re used data on HAP structure from [5], presented in the following table:
| Atom |
x |
y |
z |
Biso |
B(1,1) |
B(2,2) |
B(3,3) |
B(1,2) |
B(1,3) |
| Ca1 |
0.66667 |
0.33333 |
0.00144 |
|
0.00408 |
0.00408 |
0.0033 |
0.00204 |
0 |
| Ca2 |
-0.00657 |
0.24706 |
0.25 |
|
0.00308 |
0.00353 |
0.00417 |
0.00156 |
0 |
| P |
0.36860 |
0.39866 |
0.25 |
|
0.00223 |
0.0025 |
0.0034 |
0.00127 |
0 |
| O1 |
0.48500 |
0.32890 |
0.25 |
|
0.0036 |
0.0044 |
0.0059 |
0.0027 |
0 |
| O2 |
0.46490 |
0.58710 |
0.25 |
|
0.0035 |
0.0031 |
0.0105 |
0.0016 |
0 |
| O3 |
0.25800 |
0.34350 |
0.0703 |
|
0.005 |
0.0096 |
0.007 |
0.00505 |
-0.0025 |
| O(H) |
0 |
0 |
0.1979 |
|
0.003 |
0.003 |
0.012 |
0.0015 |
0 |
| H |
0 |
0 |
0.04 |
3.3 |
|
|
|
|
|
In the program Crystals [6] are received the *.pdb file with coordinates of all atoms (for one and several channels) and qualitative images of HAP structure. Several of the obtained results are presented on Fig.1.- 4.
As the entrance data (initial file) for Crystals software the relative coordinates x, y, z from the above given table multiplied by the appropriate parameters of a cell were used. Parameters of a cell and group of symmetry of a crystal also were set: P6/m and P63/m.
The three-dimensional model of a crystal was constructed from a *.pdb file about the help plug-in'Ю Voxel (developed in the IMPB) for Maxon Cinema 4D 8.5. The videofragments are received in discreet combustion 3. Some examples of these results are presented on Fig.2.
The detailed discussion of our last results, possible mechanisms of charge transfer and HAP polarization, changes of HAP adhesion and mechanical properties, several further steps of our investigation and demonstration of video-fragments for HAP structures will be presented on this our symposium lecture.
This work was partly supported by PERCERAMICS grant (No. NMP3-CT-2003-04937) from FP6 of EC.
References
- N. Hitmi, D. Chatain, C. LaCabanne, J. Dugas, J.C.Trombe, C. Rey and G. Montel, Solid State Communications, Vol. 33, 1980, pp. 1003-1004.
- N. Hitmi, C. LaCabanne and R.A. Young, J. Phys. Chem. Solids, Vol. 47, No6, 1986,
pp. 533-546.
- S. Nakamura, H. Takeda and K. Yamashita. Proton transport polarization and depolarization of hydroxyapatite. J. Appl. Phys., Vol.89, No.10,15 May 2001, pp. 5386-5392.
- M. Ueshima, S. Nakamura, and K. Yamashita. Huge, Millicoulomb Charge Storage in Ceramic Hydroxyapatiteby Bimodal Electric Polarization. Advanced Materials, Vol. 14, No 8, 2002, pp. 591- 595.
- J.M Hughes, M. Cameron, K.D. Crowley. American Mineralogist, Vol. 74, 1989, pp. 870-876. ftp://ftp.geo.arizona.edu/pub/xtal/data/AMCfiles/01209.amc
- http://www.xtl.ox.ac.uk/crystals.html
- http://www.gaussian.com
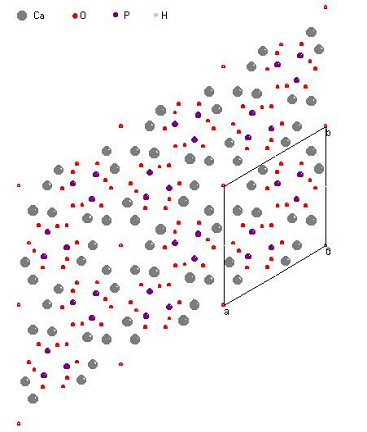 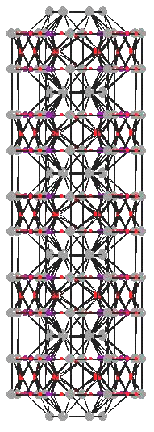
Fig.1,2. HAP structures, received in Crystals P 6/m
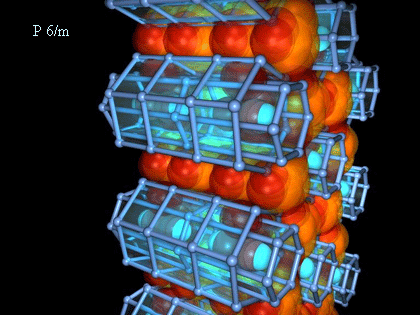
Fig.2. Tree-dimensional HAP structures and video-fragment
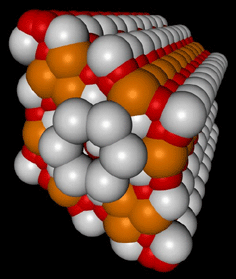 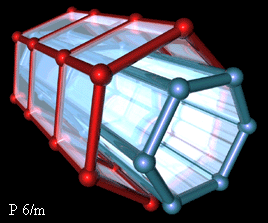
Fig 3,4. Tree-dimensional HAP structures (one channel)
|



